Palisades Therapeutics Demonstrates Breakthrough Anti-Metastatic Activity in Advanced Prostate Cancer Models
Novel Compounds Show Superior Efficacy to Standard-of-Care Enzalutamide in 5-Day Zebrafish Platform Study
PT150 could represent a first-in-class candidate with the potential to curb metastasis while mitigating hormone-therapy–associated depression.”
CLIFFSIDE PARK, NJ, UNITED STATES, October 21, 2025 /EINPresswire.com/ -- Pop Test Oncology LLC, operating as Palisades Therapeutics, today announced compelling pre-clinical results for investigational compounds Phase 2- PT150 and Pre-clinical Dimer-PT157, demonstrating potent anti-metastatic activity in AR/GR-driven prostate cancer models using the revolutionary Zebrafish Tumor Xenograft (ZTX® ONCOLEADS) Platform.— Investigators at Mount Sinai Department of Urology
The breakthrough findings, generated through a global collaboration with BioReperia AB (Sweden), Department of Urology at the Icahn School of Medicine at Mount Sinai, and leading research institutions, show that these novel therapeutics significantly outperform existing treatments in preventing cancer metastasis—the primary cause of prostate cancer mortality.
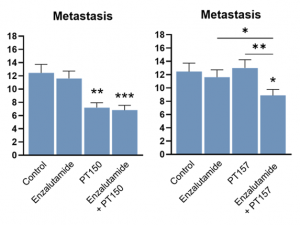
Key Breakthrough Results
PT150 delivered marked reduction in metastatic dissemination of LNCaP prostate cancer cells as a single agent, demonstrating potent stand-alone anti-metastatic efficacy that surpassed standard-of-care treatments.
PT157, a next-generation dimeric analog of PT150, achieved synergistic reduction in metastasis when combined with Enzalutamide, delivering enhanced therapeutic potential where conventional agents fail to impact metastatic progression. Beyond its synergistic antimetastatic effect with enzalutamide, PT157 also reduced primary tumor burden (data available upon request). These results indicate that next-generation PT157 merits advancement for combination therapy aimed at controlling both primary and metastatic disease.
The combination studies revealed that PT150 + Enzalutamide produced results comparable to PT150 alone, confirming PT150's central role in the anti-metastatic effect and suggesting the compound's therapeutic dominance over current standard treatments.
Critically, PT150—a proven clinical-stage antidepressant—is anticipated to mitigate the depression and suicidal tendencies associated with Enzalutamide treatment while simultaneously preventing metastasis. This dual mechanism addresses two of the most challenging aspects of advanced prostate cancer therapy: the life-threatening side effects of current treatments and the progression to metastatic disease.
Investigators at Mount Sinai believe these results are promising. PT150 is an investigational agent; its safety has been evaluated in prior clinical studies conducted by Palisades Therapeutics, and it has been explored for antidepressant effects. Emerging data suggest it may also reduce tumor progression. If confirmed, PT150 could represent a first-in-class candidate with the potential to curb metastasis while mitigating hormone-therapy–associated depression. Ongoing research is addressing this for PT150 as well as its dimer PT157.
Addressing Dual Unmet Medical Needs
The addition of PT150's established antidepressant properties to its demonstrated anti-metastatic activity creates a compelling therapeutic profile that addresses two critical challenges in prostate cancer care.
Metastatic Prevention: PT150 and PT157 demonstrate the ability to prevent metastatic dissemination—the leading cause of cancer mortality—positioning them as potentially transformative therapeutics for patients with advanced diseases.
Psychiatric Safety: Unlike current standard-of-care treatments that carry significant risks of depression and suicidal ideation, PT150's proven clinical-stage antidepressant activity offers the potential to improve rather than compromise patient mental health during cancer treatment.
Rapid Results Enable Accelerated Development
The studies utilized BioReperia's ZTX® Platform, which delivers personalized drug-response insights in just five days—a dramatic improvement over conventional mouse patient-derived xenograft (PDX) models requiring months to complete. This 10-fold speed advantage, combined with significantly lower costs, positions the platform as a transformative tool for precision oncology development. The ZTX® system achieved 100% engraftment across multiple cancer models, including difficult-to-model glioblastoma, demonstrating robust clinical relevance that traditional mouse studies often cannot match.
Addressing Critical Unmet Need
With nearly 95% of oncology drug candidates failing during clinical development primarily due to patient response variability, PT150 and PT157's demonstrated anti-metastatic activity addresses a critical gap in prostate cancer treatment. The ZTX® Platform's ability to identify responders and non-responders within days enables biomarker-based patient stratification, potentially revolutionizing clinical trial design and reducing costly late-stage attrition that plagues oncology development.
See poster at this link: https://bioreperia.com/wp-content/uploads/2025/10/Poster-ASCO-2025.pdf
About PT150 and PT157
PT150 and PT157 are investigational compounds targeting AR/GR-driven prostate cancer developed by Palisades Therapeutics. PT150 brings proven clinical-stage antidepressant activity to oncology applications, while PT157, as a dimeric analog of PT150, demonstrates enhanced synergistic activity at lower doses when combined with standard treatments, offering potential combination therapy advantages for patients with treatment-resistant disease.
About Pop Test Oncology/Palisades Therapeutics
Pop Test Oncology LLC operating as Palisades Therapeutics is a U.S.-based virtual pharmaceutical collaboration focused on innovation without infrastructure. The platform has completed over 900 studies spanning oncology, antiviral, neuropsychiatric, and metabolic disease research through its global network of over 100 scientists, clinicians, and industry professionals.
Global Research Collaboration
This study was conducted through collaboration with:
• BioReperia AB (Linköping, Sweden)
• Icahn School of Medicine at Mount Sinai (New York, USA)
• NYU Langone Medical Center (New York, USA)
• Inaphaea Biolabs (Nottingham, UK)
• Linköping University (Linköping, Sweden)
________________________________________
Forward-Looking Statements: This press release contains forward-looking statements regarding potential therapeutics. These compounds are investigational and have not been approved by any regulatory authority.
@AbbVie @Johnson-Johnson @Bristol-Myers-Squibb @Astellas-Pharma @Pfizer @Gilead @Regeneron @Ono-Pharma
#Pfizer, #Gilead, #BMS, #Astellas, #Oncology, #CancerResearch
Randi Altschul
Pop Test Oncology/Palisades Therapeutics
+1 201-943-3770
randi@poptestllc.com
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.